You put a lot of effort into protecting patients from the flu. With Flucelvax® you can provide an exact antigenic matched vaccine to the WHO-selected influenza strains.1-4†
† Does not predict a clinical effect
DESIGNED TO BE AN EXACT MATCH TO WORLD HEALTH ORGANISATION (WHO) - SELECTED STRAINS 2,4-6 †
† Does not predict a clinical effect

Effectiveness
With data in people 6 months and older, you have an advanced option for flu vaccination this season.1
*Observational studies have limitations including potential for selection bias, residual confounding. Studies conducted in different seasons with different circulating strains. Studies carried out in either trivalent or quadrivalent formulation; effectiveness results are applicable to both. + Over 5 separate US seasons (2017-20, 2022-24) and across different datasets.

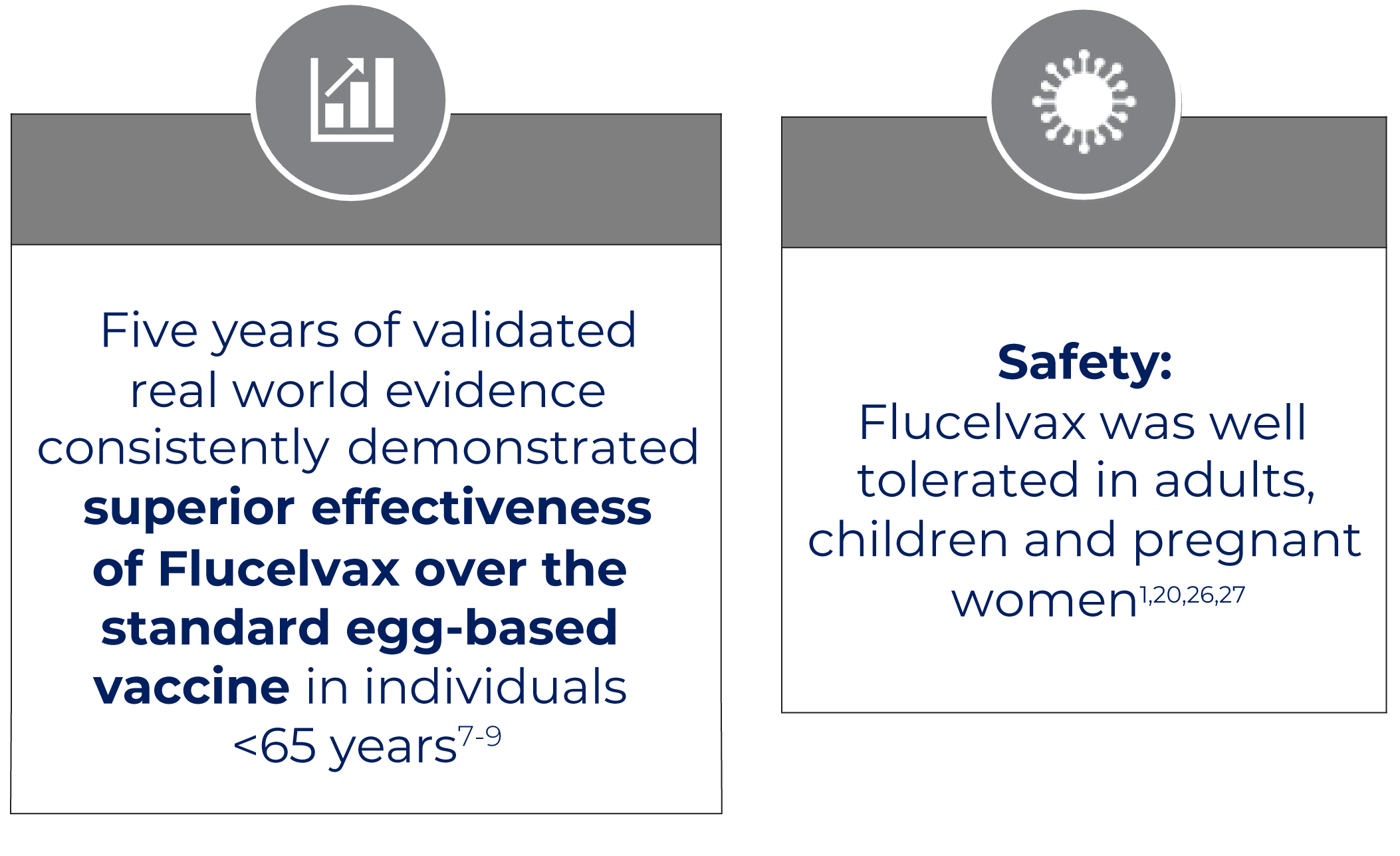
Vaccine effectiveness of Flucelvax versus egg-based influenza vaccines7-18

WATCH HOW OUR CELL-BASED TECHNOLOGY WORKS19

ESTABLISHED SAFETY PROFILE1,20-24
In clinical trials, the most commonly reported adverse events were mostly mild or moderate in intensity.1,20-24 These include:
- pain/redness at the injection site
- headache
- fatigue
- irritability (Children 6 months - 3 years)
- adverse events were tolerated with a safety profile similar to other flu vaccines
- in 2 large randomised controlled trials conducted in children (N=2,332) and adults (N=2,680) there were no serious adverse events assessed as being related to study vaccines
- Flucelvax is effective and well tolerated in pregnant women
The importance of offering your patients a choice of influenza vaccine.
Brisbane GP, Dr Sarah Chu shares her experience on offering patients a choice of influenza vaccine and reflects on her clinical experience with Flucelvax®.

Summary
Important information regarding ordering Flucelvax for 2026
Flucelvax can be ordered directly from HCL in the usual manner. Note: no minimum order quantity and no small order charge apply.
Flucelvax will cost $17.00+GST per dose from HCL, and will be available in boxes of 10 units.
References: 1. Flucelvax Data sheet October 2024. 2. Centers for Disease Control and Prevention. Cell-based flu vaccines. Accessed Dec 2025. https://www.cdc.gov/flu/prevent/cell-based.htm 3. Rockman S et al. Vaccines (Basel). 2022;11(1):52. doi:10.3390/vaccines11010052 . 4. Rajaram S et al. Ther Adv Vaccines Immunother. 2020;8:2515135520908121. doi:10.1177/2515135520908121 5. Mabrouk T et al. Dev Biol (Basel). 2002;110:125–134. 6. WHO. Influenza A(H3N2) lineage cell culture-derived CVVs for development and production of vaccines for use in the 2022 SH influenza season; Available from: https://cdn.who.int/ media/docs/default-source/influenza/cvvs/cvv-southern-hemisphere-2022/ a(h3n2)---cell-culture-derived.pdf-cell_sh21.pdf?ua=1 Accessed Dec 2025. 7. Stein AN, et al. Open Forum Infect Dis. 2024;11(5):ofae175. 8. Stein A et al. Infect Dis Ther 3/10/2025; https://doi.org/10.1007/s40121-025-01230-2 9. Stein AN et al Influenza and Other Respiratory Viruses, 2025; 19:e70180 https://doi.org/10.1111/irv.70180 10. Krishnarajah G, et al. Vaccines (Basel). 2021;9(2):80. 11. Divino V, et al. Open Forum Infect Dis. 2021;9(1):ofab604. 12. Boikos C, et al. Clin Infect Dis.2020;71(10):e665-e671. 13. Boikos C, et al. Vaccines (Basel). 2022;10(6):896 13. Eick-Cost et al. International Conference on Emerging Diseases 2018. P109. 14. Bruxvoort KJ et al. Vaccine 37 (2019) 5807-5811. 15. DeMarcus L et al. Vaccine, 2019 (30): 4015-4021. 16. Martin ET et al. J Infect Dis, 2021; 223(12): 2062–2071. 17. Stein A et al. Presentation at ESWI Influenza Conference, 2023. 18. Tseng HF et al. Options X for the Control of Influenza 2019. Abstract 10395. 19. CSL Seqirus Data on File #11. 20. Bart S et al. Hum Vaccin Immunother. 2016;12(9):2278- 2288. 21. Hartvickson R et al. Int J Infect Dis. 2015;41:65-72. 22. FDA. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ ApprovedProducts/UCM504789.pdf. Accessed Dec 2024. 23. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04074928. Accessed Dec 2024. 24. Essink BJ et al. Pediatrics. 2022. 150(5):e2022057509. 25. Seqirus Data on File #9. 26. Nolan T et al. N Engl J Med. 2021; 385:16; 27. Albano M et al. IDSOG. Virtual Poster. 2021; V130_11OB
Flucelvax® is an unfunded Prescription Medicine. Flucelvax® is an inactivated trivalent influenza vaccine, prepared in cell cultures as a suspension for injection, in a single-dose glass syringe. PRESENTATION: Each dose of Flucelvax® Quad contains 60 micrograms/0.5 mL of surface haemagglutinin and neuraminidase from four influenza virus strains. Each dose of Flucelvax® contains 45 micrograms/0.5 mL of surface haemagglutinin and neuraminidase from three influenza virus strains. INDICATIONS: For the prevention of influenza caused by Influenza Virus, types A and B, in adults and children 6 months of age and older. CONTRAINDICATIONS: Known severe allergic reaction (e.g. anaphylaxis) to a previous influenza vaccination or to any component of the vaccine. ADVERSE EVENTS: Local injection site pain, erythema and induration. Systemic headache, fatigue, myalgia, irritability, nausea, upper respiratory tract infection and nasopharyngitis. Post-marketing serious adverse events includes hypersensitivity reactions, anaphylactic shock; paraesthesia, Guillain-Barré syndrome; pruritus, urticaria, or non-specific rash; Extensive swelling of injected limb. PRECAUTIONS: Postpone immunisation in patients with febrile illness or acute infection. A protective immune response may not be elicited in all vaccine recipients, particularly in immunosuppressed patients. If Guillain-Barré syndrome has occurred within 6 weeks of previous influenza vaccination, the decision to give Flucelvax® Quad should be based on careful consideration of the potential benefits and risks. Treatment and supervision for anaphylactic reactions should be available. Co-administration with other vaccines has not been studied. DOSAGE AND ADMINISTRATION: By intramuscular injection only. Gently shake to produce a clear to slightly opalescent suspension before use. Store at 2–8°C; do not freeze; protect from light. Before prescribing, review the Flucelvax® Data Sheet (10/2024) www.medsafe.govt.nz, Seqirus Auckland, 0800 502 757. Flucelvax® is a registered trademark of Seqirus UK Ltd and its affiliates.
To report an adverse event to a CSL Seqirus product click here