WHO's your exact match?
An education module on flu and Flucelvax®
You call the shots!
An education module on flu and FLUAD®
A practical guide to discussing advanced flu vaccines with patients
Renowned New Zealand microbiologist, Ben Harris leads and chairs a preview of the 2025 influenza season. He talks about the importance of influenza vaccination an d presents an overview of the epidemiology of the 2024 season. Immunisation Coordinator and Vaccinator Assessor, Sian Gilhooley discusses the benefits of offering a choice of influenza vaccines and provides tips on how to present these options to patients. Finally we hear from Vicky Chan, a Pharmacist from Unichem Pakuranga. She relays her experience with offering flu vaccines and how she prepares for a successful flu season.
Flucelvax and the relevance of real world data to influenza.
Presentations from Dr Janine Paynter on real world or observational studies of vaccines and Professor Tino Schwarz on the clinical benefits of a cell-based influenza vaccine and his experience using Flucelvax®.
All things influenza
Presentations from Associate Professor John Litt – “What’s New with Flu?”; Registered nurses, Maree McKenna and Paige Dummond – “Managing Flu in a primary care context at Masterton Medical”; and Medical microbiology scientist, Ben Harris – “Influenza in the context of other emerging infectious diseases”.
Learn more about influenza in the elderly
Professor Stefan Gravenstein, Brown University Professor of Medicine discusses the burden of influenza in the elderly.
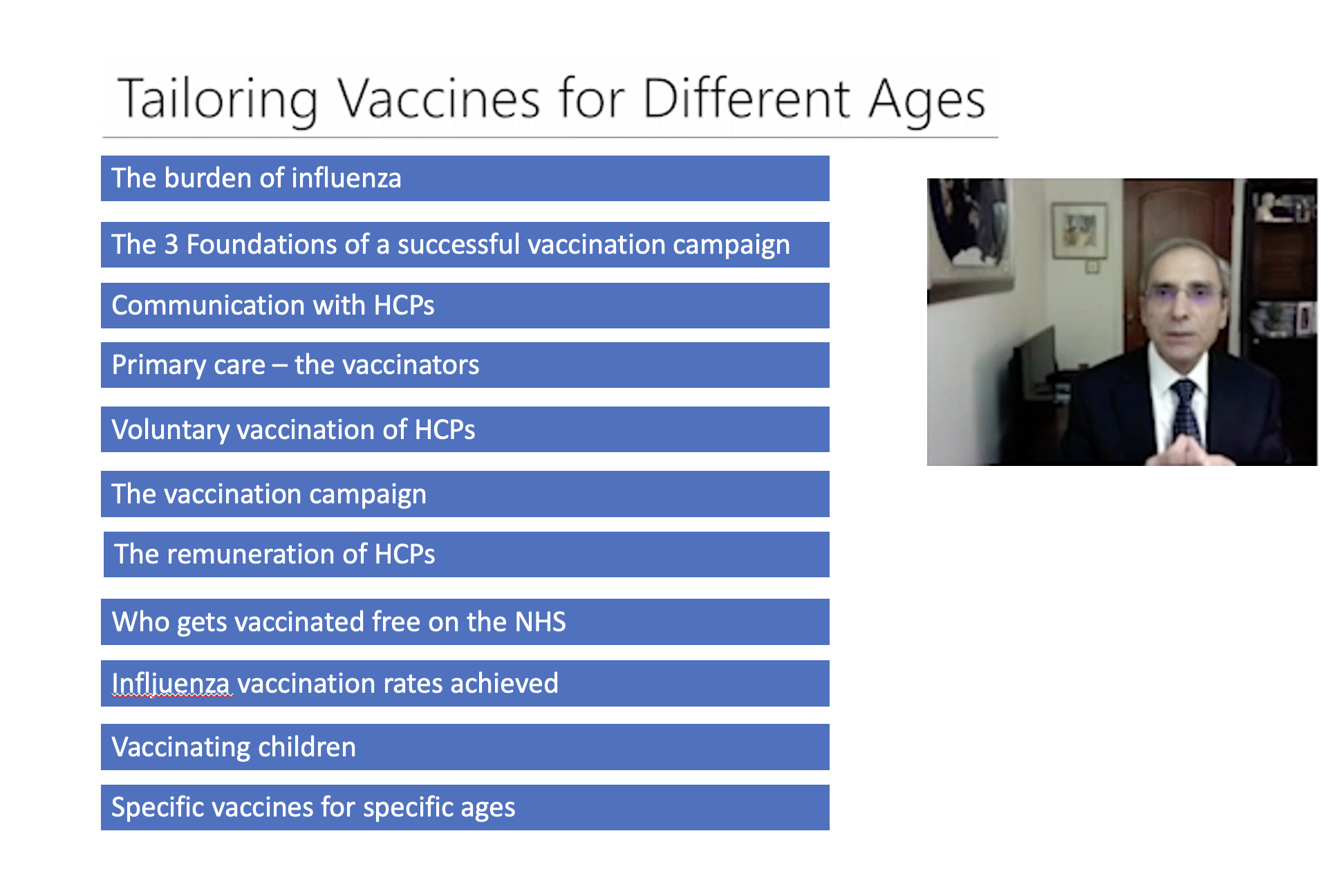
Tailoring Vaccination for Different Ages
Dr George Kassianos, the National Immunisation Lead of the Royal College of General Practitioners in the UK, and Dr Edwin Reynolds from IMAC present their thoughts on tailoring vaccination by age group.
Research Review educational series
This review summarises influenza prevention and control in the context of the attenuating effects of ageing on immunity and immune response to vaccination and discusses the history and role of adjuvants in vaccine technology, with emphasis on the use of the MF59-adjuvanted vaccine to increase influenza protection in older adults.
Flucelvax® is an unfunded Prescription Medicine. Flucelvax® is an inactivated trivalent influenza vaccine, prepared in cell cultures as a suspension for injection, in a single-dose glass syringe. PRESENTATION: Each dose of Flucelvax® Quad contains 60 micrograms/0.5 mL of surface haemagglutinin and neuraminidase from four influenza virus strains. Each dose of Flucelvax® contains 45 micrograms/0.5 mL of surface haemagglutinin and neuraminidase from three influenza virus strains. INDICATIONS: For the prevention of influenza caused by Influenza Virus, types A and B, in adults and children 6 months of age and older. CONTRAINDICATIONS: Known severe allergic reaction (e.g. anaphylaxis) to a previous influenza vaccination or to any component of the vaccine. ADVERSE EVENTS: Local injection site pain, erythema and induration. Systemic headache, fatigue, myalgia, irritability, nausea, upper respiratory tract infection and nasopharyngitis. Post-marketing serious adverse events includes hypersensitivity reactions, anaphylactic shock; paraesthesia, Guillain-Barré syndrome; pruritus, urticaria, or non-specific rash; Extensive swelling of injected limb. PRECAUTIONS: Postpone immunisation in patients with febrile illness or acute infection. A protective immune response may not be elicited in all vaccine recipients, particularly in immunosuppressed patients. If Guillain-Barré syndrome has occurred within 6 weeks of previous influenza vaccination, the decision to give Flucelvax® Quad should be based on careful consideration of the potential benefits and risks. Treatment and supervision for anaphylactic reactions should be available. Co-administration with other vaccines has not been studied. DOSAGE AND ADMINISTRATION: By intramuscular injection only. Gently shake to produce a clear to slightly opalescent suspension before use. Store at 2–8°C; do not freeze; protect from light. Before prescribing, review the Flucelvax® Data Sheet (10/2024) www.medsafe.govt.nz, Seqirus Auckland, 0800 502 757. Flucelvax® is a registered trademark of Seqirus UK Ltd or its affiliates.
FLUAD® is an unfunded Prescription Medicine. FLUAD® is an inactivated influenza vaccine, with an MF59® Adjuvant, as a suspension for injection in a single-dose glass syringe. PRESENTATION: Each dose of FLUAD® Quad contains 60 micrograms/0.5 mL of surface haemagglutinin and neuraminidase from four influenza virus strains. Each dose of FLUAD® contains 45 micrograms/0.5 mL of surface haemagglutinin and neuraminidase from three influenza virus strains INDICATIONS: For active immunisation against seasonal influenza for people 50 years of age and older. CONTRAINDICATIONS: Known severe allergic reactions to any component of the vaccine, except egg proteins; previous dose of any influenza vaccine. ADVERSE EVENTS: Common injection site pain, fatigue and headache. Most of these reactions disappear within 3 days. Rare but serious events include thrombocytopenia; lymphadenopathy; muscular weakness; allergic reactions such as anaphylactic shock, anaphylaxis; encephalomyelitis, Guillain Barré syndrome, neuritis, neuralgia, paraesthesia, convulsions; vasculitis with transient renal involvement; generalised skin reactions; and severe injection-site reactions (extensive limb swelling or cellulitis-like reactions). PRECAUTIONS: Postpone immunisation in patients with acute febrile illness or infection. Antibody responses may not be protective in all vaccinees, particularly in immunosuppressed patients. FLUAD® is for intramuscular injection use only. Persons with a history of anaphylaxis to egg should be vaccinated only in medical facilities with staff experienced in recognising and treating anaphylaxis. Co-administration with other vaccines has not been studied. If Guillain-Barré syndrome has occurred within 6 weeks of previous influenza vaccination, consider potential benefits and risks. DOSAGE AND ADMINISTRATION: Gently shake before use; inject a single 0.5 mL dose into the deltoid muscle. Store at 2–8°C; do not freeze; protect from light. Before prescribing, review the FLUAD® Data Sheet at www.medsafe.govt.nz, or from Seqirus (NZ) Ltd, Auckland. FLUAD® is a registered trademark of Seqirus UK Ltd and its affiliates. 09/25 NZ-FLU-23-0026. TAPS DA 2508ER. Updated December 2025. INSIGHT 13414
To report an adverse event to a CSL Seqirus product click here